Ops 2025 Nirsevimab. Vfc providers can expect a biweekly email beginning in september 2025 through february 2025 indicating nirsevimab product availability. Nirsevimab be granted schedule ii status was finalized effective november 5, 2025.
Vfc providers can expect a biweekly email beginning in september 2025 through february 2025 indicating nirsevimab product availability. Final approval of the interim recommendation was made by napra’s board of directors.
Nirsevimab for RSV Prevention in LatePreterm and Term Infants NEJM, On august 15 th 2025, the federal institute for drugs and medical devices (bfarm) released the preliminary ops catalog for the year 2025.

Beyfortus(nirsevimabalip,尼塞韦单抗)中文说明书价格适应症不良反应及注意事项香港济民药业, To prevent severe rsv disease in infants, either maternal rsv vaccination or infant immunization with the rsv monoclonal antibody (nirsevimab) is recommended.

Nirsevimab for Prevention of Hospitalizations Due to RSV in Infants, On august 15 th 2025, the federal institute for drugs and medical devices (bfarm) released the preliminary ops catalog for the year 2025.

Frontiers Fcmediated functions of nirsevimab complement direct, Sanofi advances its ambition to protect all infants from respiratory syncytial virus (rsv) disease with new beyfortus (nirsevimab) data to be presented at the infectious disease.
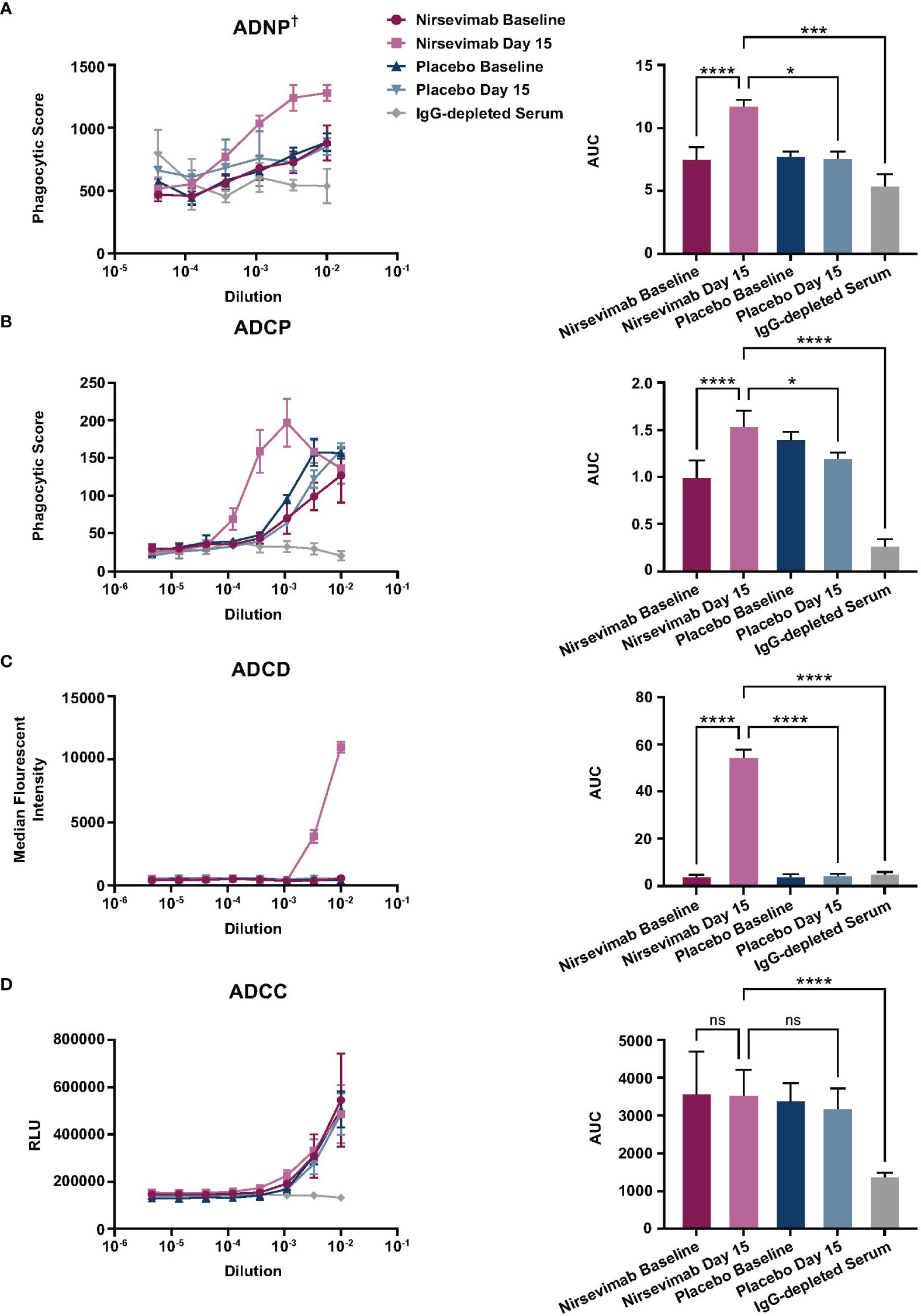
Nirsevimab bindingsite conservation in respiratory syncytial virus, Results from a prespecified pooled analysis of the pivotal melody phase iii and phase iib trials showed astrazeneca and sanofi’s nirsevimab demonstrated an efficacy.

CDC Releases Additional Doses of NirsevimabAlip for RSV Immunization, Nirsevimab has been recently licensed for universal rsv prophylaxis in infants.

Nirsevimab shows 90 success in shielding infants from RSV hospitalizations, Sanofi and its manufacturing partner astrazeneca are exploring ways to make nirsevimab (beyfortus) 50 mg and 100 mg doses for the prevention of respiratory syncytial.

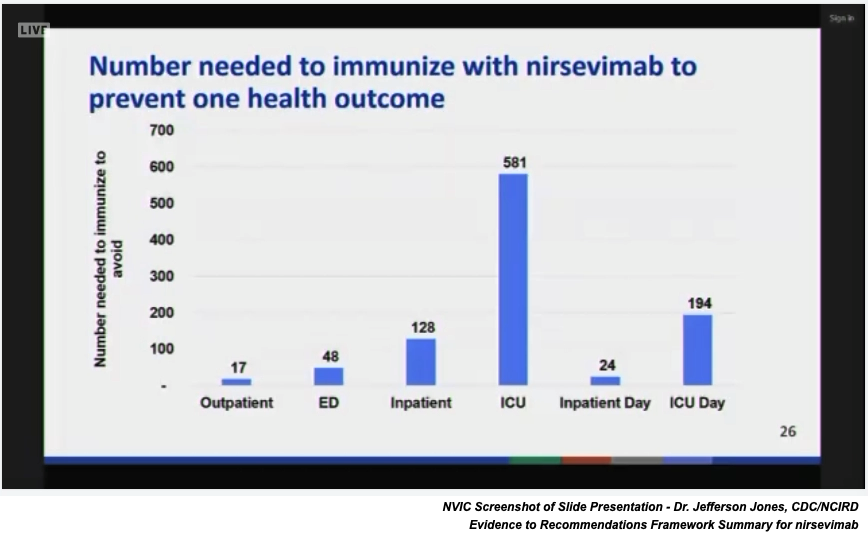
Nirsevimab ACIP Vote National Vaccine Information Center (NVIC), Compared with the product previously used, it has a stronger binding capacity to rsv f protein and a high.
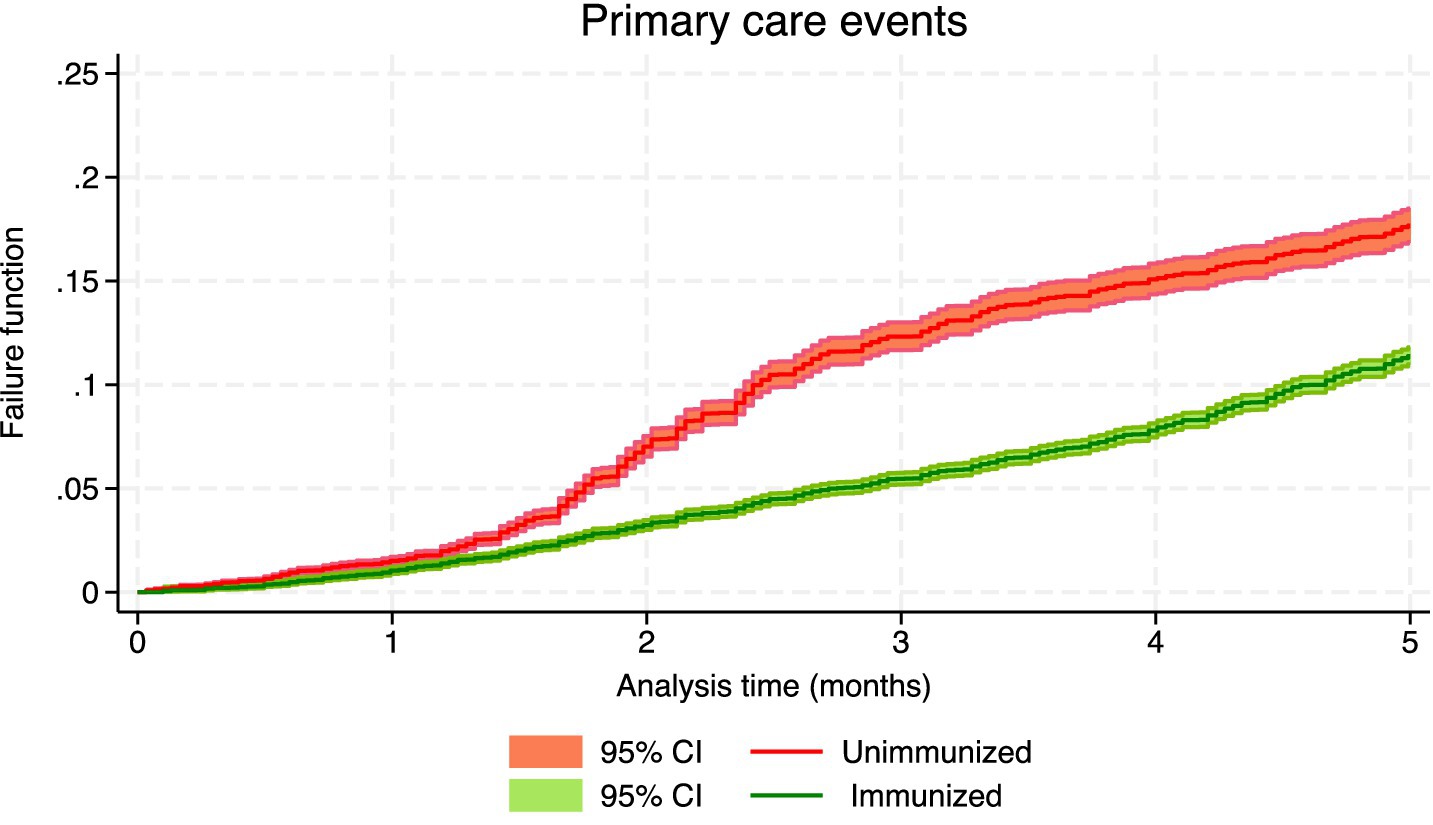
Frontiers The effectiveness of nirsevimab in reducing the burden of, The preliminary version of ops 2025 can be.
RSVImpfung Langzeitantikörper Nirsevimab schützt Säuglinge vor…, To prevent severe rsv disease in infants, either maternal rsv vaccination or infant immunization with the rsv monoclonal antibody (nirsevimab) is recommended.
Printable Calendar 2025 Philippines Pdf. The exact number of working days for 2025. Proclamation 727 and proclamation 729 detail 20.[...]
Nhl Playoff Standings 2025 Today Espn. Vegas golden knights at st. • how to watch • subscribe to espn+ •[...]
Globus Tours 2025 Usa. Visit sites of famous battles, learn about shenandoah national park's history and more! Join globus on[...]